Soap and Surfactant
- On Apr, 02, 2021
- admin
- Detergent, Surface Cleaning
Both soap and surfactant are common cleaning substances in modern life. Archeological proof has shown the existence of soap since the Babylon era, about 4800 years from now. However, surfactant only invented in 20 century with the development of chemistry and technology.
The development of surfactant becomes more rapid as the modern lifestyle has set a higher standard in personal hygiene. The variety of surfactants has improved the cleaning method for removing the contaminant effectively.
Surfactants only available within 100 years but already dominated the cleaning industry. However, soap still maintains its niche market.
The Soap
In any chemistry book, a hydrolysis process, saponification, is defined as the combination between fat and alkaline substance. The result of this process is fatty acid salt which named soap. This is a standard soap process.
If you happen to go through any hand-make soap tutorial, you can easily identify these 2 major ingredients. Other substances are added in the hand-make soap such as fragrance, those are mainly for additive purpose.
However, during the old days, the soap is generated from the fire ash (alkaline) and the kitchen cooking fat residue (fat). Hence, soap is a harsh product and mainly uses in cloth cleaning instead of skin.
Soap Characteristic
The soap comes in 2 characteristic:
- Low surface tension. The low surface tension is the general characteristic of detergent.
- Generate bubble. With agitation, the bubble generated. Even though the bubble is the by-product of any detergent solution, yet we have to properly formulate and perform bubble management so that the bubble is not overwhelming.
The deficiency of Soap
Let us repeat, the soap is a fatty acid salt from the saponification process. One of the main deficiencies of this fatty acid salt is the precipitation on the cloth.
No matter either hard or soft water, it contains several amounts of magnesium and calcium ions. The hard water, such as underground water consists even higher both ions level.
The Magnesium and Calcium ions will react with the fatty acid salt and formed a white colour stearate. The stearate or soap scum will re-deposit on the cloth and making the cloth becomes “dirty” after dry.
Soap is having poor performance under cold water and hard water condition.
Overcoming the Soap Deficiency
One of the criteria for judging a cleaning process is how effective the soil or dirt is removed compare with re-deposition. In this case, the cleaning process using “soap” may not be a good idea unless:
- Soften the water by removed the Magnesium and Calcium ions with Potassium or Sodium ions.
- Use lesser soap.
- Additional water rinsing.
The deficiency of soap has drived the development of surfactant in 20th century.
Surfactant – Main Ingredient in Cleaning Detergent
“Surface Active Agent” would be a more appropriate term for surfactant. The term already revealed that this agent is creating “active” activity on the substrate surface. In other words, the activity is referring to the cleaning effect.
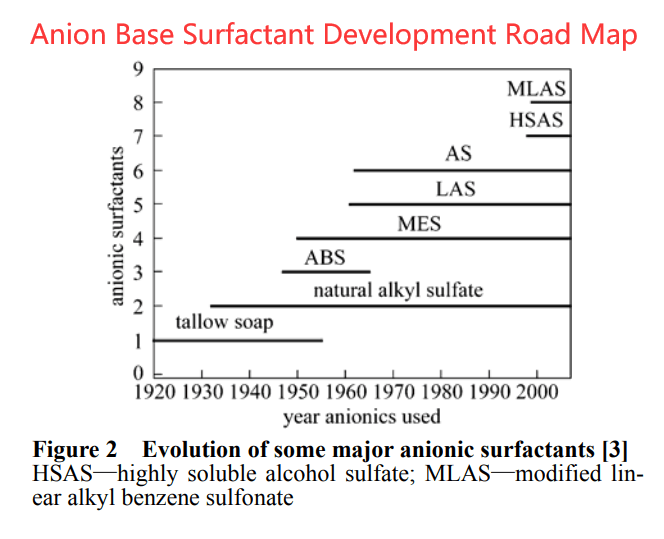
The Detergent comes into our modern life within 100 years of history. At least 15% of the detergent formulation consists of surfactant. Perhaps, this is the largest ingredient in the detergent formulation. With the development of alkylbenzene sulfonates anion surfactant would be the oldest detergent ingredient. The development of surfactant also reduces fat consumption in soap making process.
Since the surfactant is not a fatty acid salt product, hence it will not generate the stearate compound during the cleaning process. However, one of the challenges for surfactants is biodegradability. With the latest improvement in chemistry technology, most of the surfactants are having a better biodegradable ability.
Nature of Surfactant
The surfactant comes in 3 natures, namely
- Cat-ion surfactant. The cat-ion surfactant mostly referring to the group carrying a positive Nitrogen(N) positive charge ion. The cation surfactant has shown higher aquatic toxicity than other surfactants. However, the Quart-based surfactant gives a better cleaning and shorter drying time. Another characteristic is reducing the antistatic effect, which means reducing in friction force between the fabric. Hence, this surfactant normally used in the fabric softener ingredient.
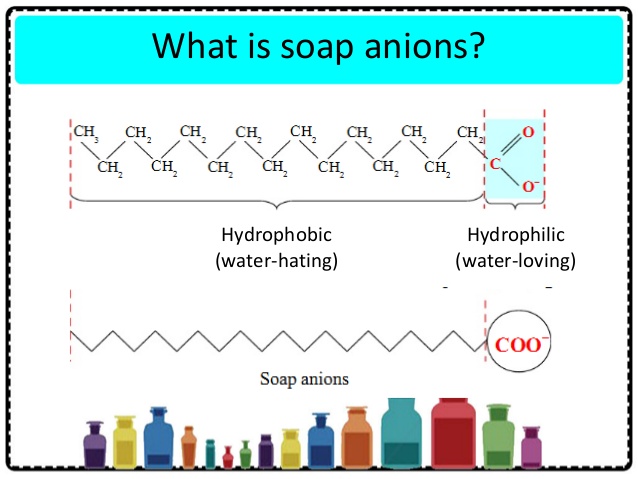
- An-ion surfactant. This is the main surfactant used in the detergent industry, such as the LAS, and it improves MLAS group. The surfactant group Mainly SLS ester
- Non-ion surfactant. The advantage of nonionic surfactant is not sensitive to hard water. In other words, no precipitation occurs. Most of this surfactant is used for animal fur fabric cleaning, such as wool cloth. The common surfactant group includes AE, MEE groups.
- Silicone surfactant. The silicone-based surfactant is mostly used in cosmetic formulation. This is to provide the silk-like feel effect. However, in industrial cleaning, silicone-based surfactant is hardly used.
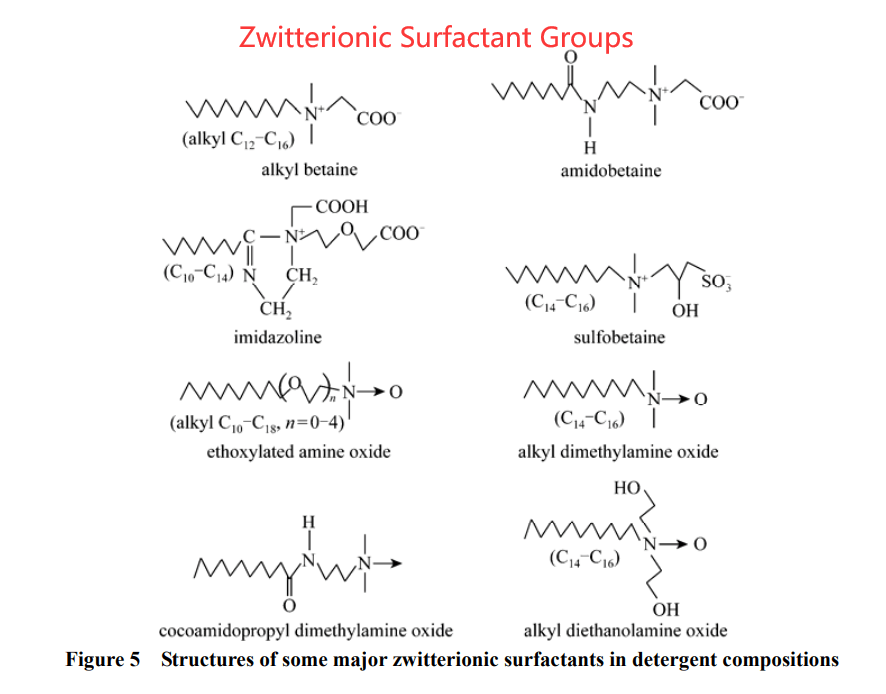
- Zwitterionic surfactant. This type of surfactant holds 2 types of ions. The amine group surfactant is the representative of this zwitterionic surfactant.
How the Detergent / Surfactant Works
The surfactant in the detergent is the core which determines the cleaning result. There are a few cleaning mechanisms that happen during the cleaning process.
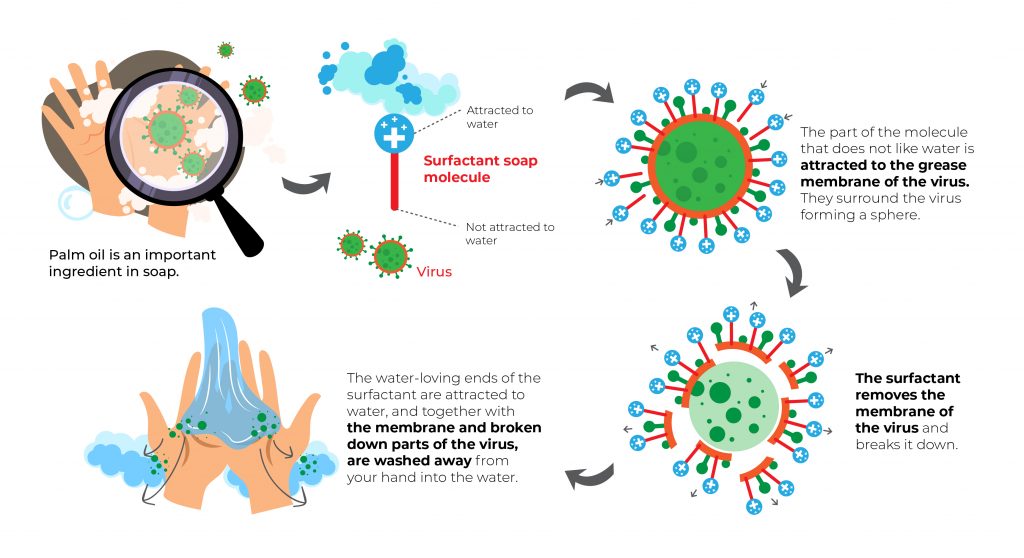
The Bi-polar Pulling Effect.
During the cleaning process, one end of the bi-polar surfactant will combine the dirt or soil and the other end will hold with a water molecule. Through the agitation force during the cleaning process, the dirt will be removed from the cloth.
Lower Surface Tension
We have mentioned the effect of surface tension in many of our articles. The surfactant mostly having lower surface tension is loosen the oil and dirt on the cloth. This will promote better rinsing results.
Ion Replacement or Chemical Reaction
Ion exchange happens while the surfactant gets in touch with the contaminant particle. Furthermore, oxidation may occur which even enhances the cleaning effect.
The Development of Surfactant
Even though there are already many kinds of surfactants available in the market, however, the development of surfactants is still an on-going process. This also shows the improvement in chemical engineering.
The development of surfactants mainly focus on several topics:
- Economical consideration.
- Environmental consideration. Develop eco-friendly and better biodegradability performance.
- Cleaning performance. Better detergency.
So far, we have revealed substance information about the surfactant. However, comes to actual detergent formulation, we practice the use of a combination of surfactants group instead of a single surfactant. The surfactant combination is to fulfill several requirements and also balance the overall detergent performance.
So far, we are seeing a smooth drift from soap to detergent application with the technology improvement from surfactant. We are looking forward to the development of surfactant, this will give us more freedom in terms of the detergent formulation.
Feel free to contact DST if you have any requirements in your cleaning process. We are here to work with you on your cleaning needs.






Leave a Comment